Abstract
Background: Splenomegaly is a marker of disease progression in MF; larger spleen size (SS) is correlated with poorer survival and debilitating MF symptoms. In a retrospective real-world study of 408 patients (pts) with MF treated with ruxolitinib (RUX), pts with SS ≥ 10 cm below the left costal margin (LCM) were significantly less likely to attain a spleen response than pts with SS < 10 cm (Palandri 2017); this finding has not been reported in clinical trials of RUX.
FEDR is a JAK2-selective inhibitor approved in the US, EU, Canada, and elsewhere as first-line (1L) MF treatment (Tx) and in pts previously treated with RUX. FEDR was evaluated as 1L MF Tx in the phase 3, placebo (PBO)-controlled JAKARTA trial, and in the phase 2, single-arm JAKARTA2 trial in pts previously treated with RUX. Pts allocated to FEDR 400 mg/day (d) in these trials reported substantial reductions from baseline (BL) in spleen volume and MF symptom severity after 6 FEDR Tx cycles. It is important to determine whether spleen volume and symptom improvements during FEDR Tx are influenced by degree of pre-Tx splenomegaly.
Objective: Investigate the clinical efficacy of FEDR 400 mg/d in pts in JAKARTA & JAKARTA2 with large spleens at BL.
Methods: The JAKARTA trial assessed FEDR 400 mg/d, FEDR 500 mg/d, and placebo in pts with JAK-inhibitor-naïve MF, and the JAKARTA2 trial evaluated FEDR 400 mg/d (starting dose) in pts resistant/intolerant to prior RUX. Both studies enrolled pts with intermediate- or high-risk MF, SS > 5 cm from LCM by palpation, platelets ≥ 50 ×10 9/L, and ECOG PS ≤ 2. These analyses include pts in each trial allocated to receive FEDR 400 mg/d in continuous 28d Tx cycles.
Too few pts had BL SS < 10 cm by palpation in JAKARTA (n = 21) and JAKARTA2 (n = 12) for meaningful comparison vs SS ≥ 10 cm. Thus, in these post hoc analyses we divided pts according to the median SS (mSS) at BL in each study , and assessed changes from BL in spleen volume and MFSAF total symptom score (TSS) at the end of cycle 6 (EOC6). Spleen volume was assessed by MRI/CT scan; MFSAF TSS was the sum of 6 MF symptom scores: night sweats, early satiety, pruritus, pain under ribs-left side, abdominal discomfort, and bone/muscle pain. Pts must have had spleen volume and/or TSS data at BL and EOC6.
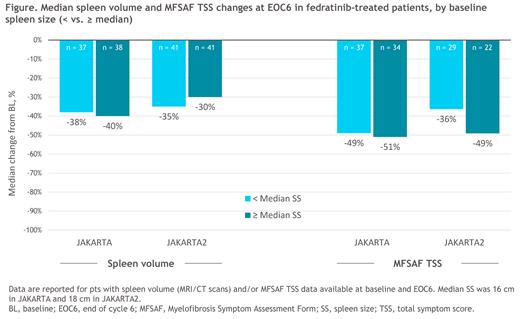
Results: In JAKARTA, 96 pts were randomized to FEDR 400 mg/d; mSS at BL for all pts was 16 cm (range 5-40). Median (range) BL spleen volume was 1771 (316-3396) mL in the < mSS cohort and 3329 (983-6430) mL in the ≥ mSS cohort; median TSS was 13.0 and 17.6, respectively. Spleen volume data were available at BL and EOC6 for 37 (82%) and 38 (75%) pts in the < mSS and ≥ mSS cohorts, respectively. Median spleen volume reduction (SVR) at EOC6 was -38% [95%CI -43%, -31%] in the < mSS group and -40% [-40%, -28%] in the ≥ mSS group (Figure). Changes in MF symptom scores at EOC6 were similar in the < mSS (n = 37) and ≥ mSS (n = 34) cohorts, with median TSS reductions of -49% and -51%, respectively. For individual symptoms, median reductions in abdominal discomfort, bone/muscle pain, and early satiety were similar between BL SS cohorts, whereas changes were greater in the < mSS group for pruritus and pain under ribs-left side, and greater in the ≥ mSS group for night sweats.
In JAKARTA2, 97 pts received FEDR 400 mg/d (starting dose); BL mSS for all pts was 18 cm (range 5-36). Median (range) BL spleen volumes were 1937 (737-4305) and 4002 (1821-7815) mL for pts with < mSS and ≥ mSS, respectively; and BL TSS was 17.2 (0.7-48.0) and 23.6 (1.0-44.0). Spleen volume and MFSAF TSS data were available at BL and EOC6 for 41 and 29 pts, respectively, in the < mSS cohort, and for 41 and 22 pts in the ≥ mSS group. At EOC6, median SVR from BL was -35% [95%CI -40%, -29%] for pts with < mSS at BL and -30% [-37%, -17%] for pts with ≥ mSS (Figure). Median TSS changes were -36% and -49% in the < mSS and ≥ mSS cohorts, respectively; pts with ≥ mSS at BL had similar or greater reductions from BL in 5/6 MFSAF symptoms (all except pruritus) vs those with < mSS.
Conclusions: These analyses demonstrate that extent of splenomegaly at BL in JAKARTA and JAKARTA2 did not influence the likelihood of achieving improvements in spleen volume or MF symptom severity for pts receiving FEDR 400 mg/d as 1L MF Tx or as post-RUX salvage Tx. For pts who completed 6 FEDR Tx cycles, reductions in spleen volume and TSS at EOC6 in the ≥ mSS cohorts in both studies were similar to those in the < mSS cohorts, even though BL spleen volumes and TSS were higher in the ≥ mSS groups.
Kiladjian: Incyte Corporation: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Taiho Oncology, Inc.: Research Funding; PharmaEssentia: Other: Personal fees; AOP Orphan: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees. Passamonti: BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AbbVie: Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Vannucchi: BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees. Talpaz: Imago: Consultancy; Celgene: Consultancy; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Constellation: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Other: Grant/research support . Cervantes: Bristol Myers Squibb: Consultancy, Honoraria. Harrison: Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Constellation Pharmaceuticals: Research Funding; Shire: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Keros: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte Corporation: Speakers Bureau; Sierra Oncology: Honoraria; Promedior: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AOP Orphan Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Galacteo: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Geron: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Mesa: AOP: Consultancy; La Jolla Pharma: Consultancy; Sierra Oncology: Consultancy, Research Funding; Incyte Corporation: Consultancy, Research Funding; Genentech: Research Funding; Samus: Research Funding; CTI: Research Funding; Celgene: Research Funding; Abbvie: Research Funding; Constellation Pharmaceuticals: Consultancy, Research Funding; Novartis: Consultancy; Gilead: Research Funding; CTI: Research Funding; Pharma: Consultancy; Promedior: Research Funding. Mascarenhas: Geron: Consultancy; Merus: Research Funding; Geron: Consultancy, Research Funding; Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Constellation: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Prelude: Consultancy; Galecto: Consultancy; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; CTI Biopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Forbius: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Promedior: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech/Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Verstovsek: Promedior: Research Funding; CTI BioPharma: Research Funding; Blueprint Medicines Corp: Research Funding; Protagonist Therapeutics: Research Funding; Genentech: Research Funding; Ital Pharma: Research Funding; Incyte Corporation: Consultancy, Research Funding; Gilead: Research Funding; NS Pharma: Research Funding; PharmaEssentia: Research Funding; Celgene: Consultancy, Research Funding; Roche: Research Funding; AstraZeneca: Research Funding; Novartis: Consultancy, Research Funding; Sierra Oncology: Consultancy, Research Funding; Constellation: Consultancy; Pragmatist: Consultancy. Devos: Alexion, AstraZeneca Rare Disease Inc.: Consultancy; Incyte: Consultancy; AbbVie: Consultancy; Bristol Myers Squibb - Celegene: Consultancy; Novartis: Consultancy. Rose: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Zhang: Bristol Myers Squibb: Current Employment. Sy: Bristol Myers Squibb: Current Employment.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal